Interference Reflection Microscopy (IRM) uses reflected polarised light to study cellular interfaces on a glass surface without using fluorescent labels. Confocal microscope lasers are polarised, which makes them ideal for IRM. You can acquire fluorescence images at the same time as reflection images but with the White Light Laser (WLL) on the LMCB SP8 a notch filter will be needed to stop reflected light being detected in the fluorescence channels.
Procedure
Select a detector for your reflection image. It would probably be best to use a PMT since the laser light reflected off the coverslip could well be too bright for a HyD. Keep the gain reasonably low to avoid saturation
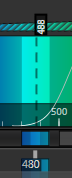
Set the detection wavelengths so the laser is reflected into the detector. The image below shows a typical configuration where the 488 laser (dotted line) is being reflected into a band between 480 and 495 nm
Click the Acousto-Optic Beam Splitter (AOBS) button
Set the AOBS Configuration to Reflection for the lines you want to reflect
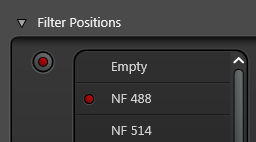
If you want to scan a fluorescence channel as well then you have to image in Sequential mode, otherwise you will also see some light reflecting into the fluorescence channel. Reflections can be suppressed in the fluorescence channel by selecting a notch filter position in the Fluorifer Disc to block the laser. Click the Fluorifer Disc button
Pick a suitable notch filter to block the lasers you are using. For example, NF 488 will block the 488 nm laser
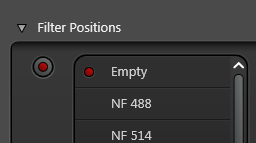
The notch filter position must be empty in the reflection channel or you won't see any reflection
You must set the Sequential Scan to Between Frames or Between Stacks as the Fluorifer Disc cannot rotate quickly enough to switch between channels between lines