Confocal Microscopes
Confocal Microscopy - An Introduction
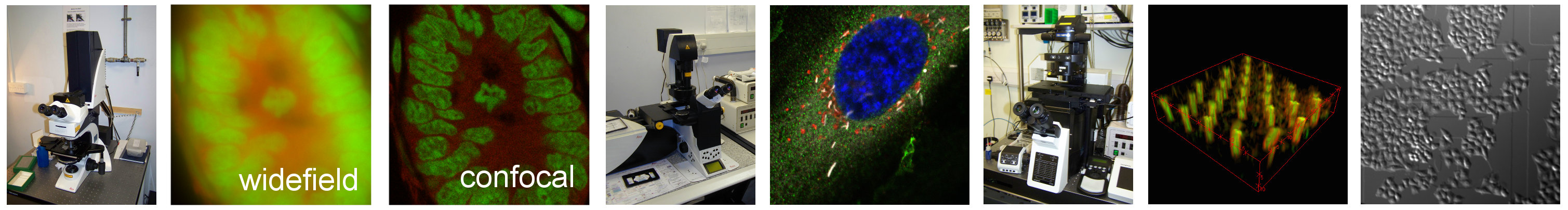
In wide-field microscopy the contrast of the focused image can sometimes be reduced by light from out of the focus plane - a phenomenon called blur. This is especially problematic for thick fluorescent specimens such as tissue or small organisms. Confocal microscopes are designed to reduce the amount of out-of-focus light reaching the detector.
Laser scanning confocal microscopes illuminate very small volumes within the sample with a focused laser spot so that the entire sample is not illuminated at the same time. The light emitted from the spot travels back through the microscope and through a pinhole in front of the detector. This pinhole is in a conjugate plane to the focused laser spot in the sample plane, so an image of the spot is formed at the pinhole. Light from above and below the plane of focus will come into focus above and below the pinhole plane and most will not go through the pinhole. So most of the light reaching the detector is from the focused spot.
Since a confocal image represents a single ‘slice’ from a three-dimensional sample with next to no light coming from adjacent slices, a motorised focus mechanism can be used to acquire every slice from the sample in sequence. These slices can then be reconstructed into a stack that represents the entire 3D structure. This is called optical sectioning.
Another technique called deconvolution can be used to computationally reassign light back to its correct plane of focus in both wide-field and confocal images by applying information known about how the microscope distorts the image of the relative to the object (the point spread function) to a reconstruction algorithm.
Confocal laser scanning microscopes (CLSM)
Most of the confocal microscopes in the LMCB use a standard design first developed in the 1980s in which a single laser beam is deflected off galvanometer driven mirrors and scanned across the specimen in a raster pattern. Most confocal microscopes still use this design for scanning the specimen - and if someone talks about a 'confocal microscope' then most of the time this is the kind of device they will be referring to. This design is usually referred to in technical and methods papers as a confocal laser scanning microscope (CLSM) although it would be less ambiguous to call it a single point confocal laser scanning microscope. The CLSM design allows for precise adjustment of the pinhole to reject out-of-focus light and free adjustment of the optical zoom to set the optimal pixel size for sampling. These factors mean that the CLSM gives the best resolution over the widest range of objective lens magnifications and numerical apertures of all the various confocal designs. It also makes the design extremely flexible. The main disadvantage is slow speed - since it takes a long time to scan a single laser spot over a specimen, especially at high resolution.
Spinning disc confocal microscopes (SDCM)
The main purpose of spinning disc confocal is to increase the speed of capture of optically sectioned images by illuminating multiple spots on the specimen in parallel rather than having a single laser spot. The Yokogawa CSU-X1 disc rotates at a rate of 5000 rpm so an entire field of view is fully scanned in about 40 ms. Compare this to the approximately 1 second it takes to scan a similar field of view on a CLSM. Scan-for-scan SDCMs are also more gentle on the specimen and produce a higher signal-to-noise ratio than equivalent CLSM images but as always there are drawbacks. Firstly, the disc itself has thousands of pinholes drilled into it that are of a fixed size and therefore only give optimal resolution for objective lenses of the highest numerical aperture - you can't adjust the pinhole size like you can on a CLSM. Secondly, the detector is a camera with a fixed pixel size which limits resolution unless you use high magnification lenses - there is no way to optically 'zoom' on the specimen without using higher magnification lenses. Both of these points mean the SDCM is less flexible than the CLSM. Thirdly, the close spacing of the pinholes on the disc causes a phenomenon called 'pinhole crosstalk' where light from out of the plane of focus can contaminate the optical section by going through adjacent pinholes - this means the SDCM optical sectioning is worse than CLSM.